Scientist
Cambridge Display Technology
Biography
I’m a scientist at Cambridge Display Technology developing the next generation of organic electronic materials. I am a highly-motivated chemist with a strong aptitude for research and an enthusiastic interest in understanding how things work. My current research lies in the field of molecular electronics and automation science, but am always eager to broaden my knowledge into new areas.
Download my CV
- Functional Organic Molecules
- Laboratory Automation
- Organic Electronics
- Medicinal Chemistry
- Supramolecular Chemistry
DPhil in Organic Chemsitry, 2022
University of Oxford
MChem (Hons), 2018
Durham University
Skills
Experience
Cambridge Display Technology is the European R&D centre for Sumitomo Chemical.
My role accelerates the early-stage R&D of functional organic materials. I have strong experience in designing and synthesising and analysing new materials (small molecules and polymers) and working in an interdisciplinary team of scientists and engineers. While my role is primarily based around synthetic organic chemistry, I heavily participate in the development of early-stage (proof-of-concept) projects, in laboratory automation and in the preparation of IP filings.
PhD Thesis: “Towards Polyyne Rotaxanes and Catenanes” Development of a new alkyne masking group towards extended polyynic systems and using masking groups and supramolecular chemistry to generate carbon-rich rotaxanes and catenanes.
Training & Experience:
- NMR: 1D & 2D experiments, variable temperature, DOSY and time-dependent studies
- Mass Spectrometry: LC-MS (ESI), GC-MS and MALDI
- Quantum chemical calculations (Gaussian/ORCA)
- Group IT officer (data backups, website & ELN management)
- Attended the University of Oxford intensive course in XRD
Responsibilities included:
- A summer placement supporting the R&D of organic polymers for emissive and transport layers in flexible OLED devices.
- Developed standard protocols for flow-based cycloadditions, Grignard/lithiations and Ullmann chemistry.
Masters Thesis: “Synthesis and spectroscopic study of lanthanide complexes”
Organic synthesis of lanthanide complexes for use as solvent polarity probes, observed by optical and NMR spectroscopic methods.
Featured Publications
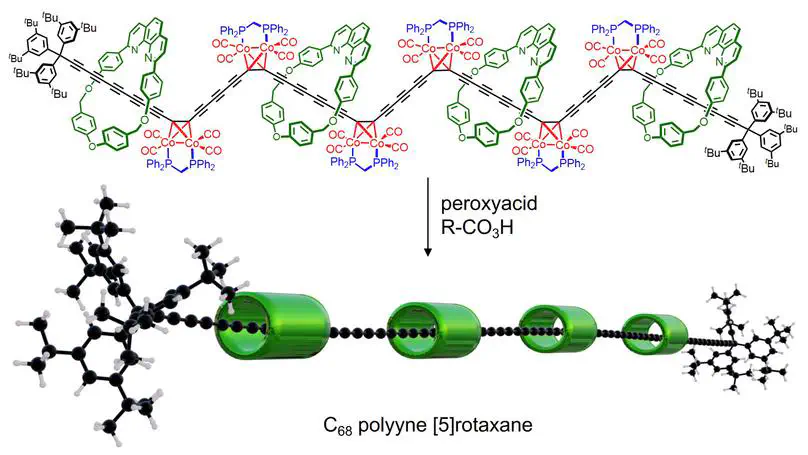
Polyynes are chains of sp 1 carbon atoms with alternating single and triple bonds. As they become longer, they evolve towards carbyne, the 1D allotrope of carbon, and they become increasingly unstable. It has been anticipated that long polyynes could be stabilized by supramolecular encapsulation, by threading them through macrocycles to form polyrotaxanes—but, until now, polyyne polyrotaxanes with many threaded macrocycles have been synthetically inaccessible. Here we show that masked alkynes, in which the C≡C triple bond is temporarily coordinated to cobalt, can be used to synthesize polyrotaxanes, up to the C 68 [5]rotaxane with 34 contiguous triple bonds and four threaded macrocycles. This is the length regime at which the electronic properties of polyynes converge to those of carbyne. Cyclocarbons constitute a related family of molecular carbon allotropes, and cobalt-masked alkynes also provide a route to [3]catenanes and [5]catenanes built around cobalt complexes of cyclo[40]carbon and cyclo[80]carbon, respectively.
Recent Publications
Contact
Feel free to contact me using the form below: